BIOS: Nucleus of Life Science Innovation 🚀
JOBS
BIOS Talent: Find Jobs @ Breakout TechBio Startups — Search Jobs 🚀
Post Jobs: Add Your Startup to BIOS Talent — Post Now 🎉
Students: Join Alix Ventures Fellowship — Join Now 🧬
BIOS Contributor: Share Your Thought Leadership — Join Now🔬
CONTENT & COMMUNITY
BIOS Daily: Join 25K+ Subscribers Following TechBio — Sign Up 🔥
BIOS Insider: Premium TechBio Thought Leadership — Sign Up ✨
BIOS Commons: World’s Largest #TechBio Community — Join Now 🎉
INVEST
BIOS Angels: 1st TechBio Angel Investing Syndicate — Join Now 🌟
Alix Limited: Invest in Breakout TechBio Startups — Learn More 🧠
By:
Alix Ventures: Supporting Early Stage Life Science Startups Engineering Biology to Drive Radical Advances in Human Health
Introduction
Since its initial discovery as a genetic reprogramming tool in 2012, CRISPR has widely been regarded as a breakthrough scientific discovery and quickly become one of the hottest new biotechnologies in the industry.
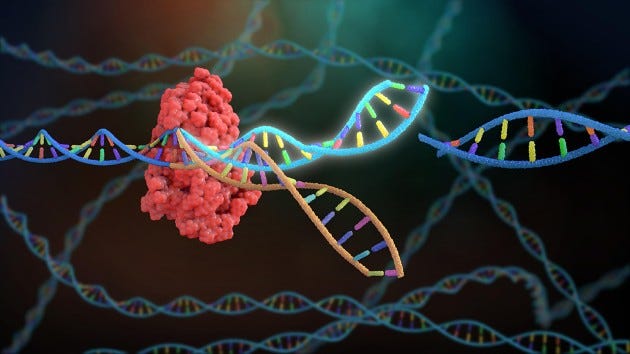
CRISPR/Cas gene editing technology arose from a bacterial defense mechanism against viral infections. It involves a guide RNA specific to a certain nucleic acid sequence, that leads a Cas protein to the site at which it binds. Binding sites must be accompanied by protospacer adjacent motifs (PAMs), which are extra base pairs in the DNA that the Cas protein needs to recognize in order to cut. Once the guide RNA binds to the host cell DNA, the Cas protein cuts the strands apart, allowing free nucleotides or other local DNA sequences to fill in the gap via endogenous DNA repair mechanisms. The guide RNA can be synthesized specifically to bind to any sequence, thus allowing scientists to directly target certain genes or genetic defects.
Therapeutically, CRISPR can be used to knock out, repair, or insert genes into the genome of the host cell. Gene knockouts are the easiest task, as only the basic CRISPR machinery is required. The guide RNA will lead the Cas protein to the location to induce a break in the DNA, where afterwards random nucleotides will fill in the gap and deform the resulting proteins that are formed during translation. This random filling in of nucleotides relies on an endogenous repair mechanism called non-homologous end joining (NHEJ), which is highly efficient but introduces insertions or deletions that can shift the entire strand. A separate endogenous mechanism known as homology-directed repair (HDR) is far less efficient and also generates unwanted insertions or deletions, but has the potential to also insert larger strands of DNA if readily available. Ongoing CRISPR development is aimed at increasing the efficiency and frequency of HDR as a ‘paste’ gene editing function.