TechBio Guide💊: Therapeutic Development - Target to Market
A step by step guide detailing the process for developing new Therapeutics
BIOS: Nucleus of Life Science Innovation 🚀
JOBS
BIOS Talent: Find Jobs @ Breakout TechBio Startups — Search Jobs 🚀
Post Jobs: Add Your Startup to BIOS Talent — Post Now 🎉
Students: Join Alix Ventures Fellowship — Join Now 🧬
BIOS Contributor: Share Your Thought Leadership — Join Now🔬
CONTENT & COMMUNITY
BIOS Daily: Join 25K+ Subscribers Following TechBio — Sign Up 🔥
BIOS Insider: Premium TechBio Thought Leadership — Sign Up ✨
BIOS Commons: World’s Largest #TechBio Community — Join Now 🎉
INVEST
BIOS Angels: 1st TechBio Angel Investing Syndicate — Join Now 🌟
Alix Limited: Invest in Breakout TechBio Startups — Learn More 🧠
By:
Alix Ventures: Supporting Early Stage Life Science Startups Engineering Biology to Drive Radical Advances in Human Health
Background
The modern drug regulatory path began in the early 1960s following the thalidomide tragedy. Designed to prevent morning sickness, thalidomide was released in 1959 and resulted in over 10,000 children in 46 countries being born with birth defects.
In the wake of thalidomide’s disastrous side effects, the World Health Organization (WHO) set up the Programme for International Drug Monitoring (PIDM) in 1968 to ensure that potential evidence of harm to patients was collected from as many sources as possible, enabling participating countries to be alerted to patterns of emerging harm across the world. Today, PIDM has more than 150 participating countries and, as of October 2021, there were over 28 million Adverse Event Reports (ADRs) collected.
In the United States…
Today, the Food and Drug Administration is the accepted gold standard for drug approval safety testing. Before commercialization, therapeutic interventions must undergo a rigorous evaluation of safety, quality, and effectiveness. But it wasn’t always this way.
The path to drug safety regulation began in 1906 when Congress passed the Pure Food and Drugs Act, which prohibited misbranded and adulterated food, drinks, and drugs in interstate commerce and was originally enforced by the Bureau of Chemistry in the Department of Agriculture. In 1930, the Bureau became the Food and Drug Administration (FDA).
However, the law only required that drugs meet standards of strength and purity. Instead, the burden of proof was on the government to show that a drug’s labeling was false and/or misleading; there was no requirement that any information be submitted to the FDA before marketing.
The Food, Drug, and Cosmetic Act of 1938 was passed after 107 people died from a poisonous ingredient in Elixir Sulfanilamide. For the first time, manufacturers were required to submit an application to the FDA showing that a drug was safe before it could be marketed. If the FDA didn't act on the application in a certain time period, the application automatically became approved.
As the WHO was responding to the tragedy of thalidomide, US public interest was rekindled in drug regulation as news reports surfaced about how FDA Medical Officer Frances O. Kelsey, M.D., Ph.D., had kept the drug thalidomide off the US market. Under this renewed scrutiny, clinical pharmacologists were called on to explain the difference between well-controlled studies and the typical drug study. With thalidomide providing the opportunity to drive change, Congress passed the Kefauver-Harris Drug Amendments to the Federal FD&C Act in October 1962. Before marketing a drug, firms now had to prove not only safety, but also provide substantial evidence of effectiveness for the product's intended use. Critically, the 1962 amendments also required that the FDA specifically approve the marketing application before the drug could be marketed.
These changes were the start of a wave of regulation designed to ensure reliable evidence of drug safety and efficacy, as well as chemical and biological purity prior to market release.
While a lack of clinical efficacy is the major cause of drug attrition, a poor safety profile is perhaps an even more significant factor in the failure of drugs during development. This attrition may occur at any stage in the development process, from initial drug discovery and preclinical trials, to clinical trials and post-marketing surveillance (pharmacovigilance).
Below, we begin to summarize and define the steps taken by biopharmaceutical companies and the FDA in the discovery, development, and approval process on the path to therapeutic commercialization.
Outline
Drug Discovery
Target
Selection
Validation
Hit Identification
High-Throughput Screening
Assay Development
Drug Development
Lead Optimization
Medicinal Chemistry
Initial Animal Efficacy Studies
In Vitro & In Vivo ADME
Pharmacology
Preclinical
Candidate Selection & Profiling
Animal Efficacy
DMPK/ADME (Drug Metabolism and Pharmacokinetics/ Absorption, Dilution, Metabolism, Excretion)
(Initial) Formulation & Delivery
GMP (Good Manufacturing Practices) Synthesis / Manufacturing
(Preliminary to Definitive) Rodent Toxicology / Genotoxicology
IND Enabling Studies
Pharmacokinetics (PK) (higher species)
Pharmacodynamic (PD) (Higher Species)
Toxicology Assessments (Higher Species)
IND Submission (Investigational New Drug)
Pre-IND Consultation Program
SEND (Standard Exchange of Nonclinical Data)
Types of Filing
Commercial
Research (Non-Commercial)
IND types
Investigator
Treatment
Emergency Use
Elements of the Application
Animal Pharmacology & Toxicology Studies
Clinical Protocols & Investigator Information
Data From Any Prior Human Research
Clinical
Phase1 - Safety & Dosing
Phase 1a: Dose Escalation → MTD
Phase 1b: Dose Expansion
Phase 2 - Efficacy & Side Effects → Optimal Dosing
Phase 1b/2a - Optimal dosing strategies
Phase 2a - Safety, tolerability, pharmacokinetics, & pharmacodynamics
Phase 2b - Preliminary efficacy studies
FDA Consultation for Phase 3 trial design
Phase 3 - Comparative Efficacy & ADE Monitoring
Phase 2b/3a
Phase 3a
Non-inferiority trials
Long-term safety studies
Pivotal Trial
NDA Preparation (New Drug Application)
Regulatory Pathway
There are 3 Major Pathways:
505(b)(1) or “Stand-alone” NDA
505(j) or abbreviated NDA (ANDA)
505(b)(2) or “Hybrid Application”
Expedited Development and/or Review
Priority Review
Breakthrough Therapy
Accelerated Approval
Fast Track
Elements
Drug Ingredients
Animal Studies
Clinical Trials
Manufacturing, Processing, & Packaging
Phase 3b
Commercial
Approval & Commercialization
Pricing
Marketing
Distribution
Phase 4 - Observational Study (Post Marketing Safety & Efficacy)
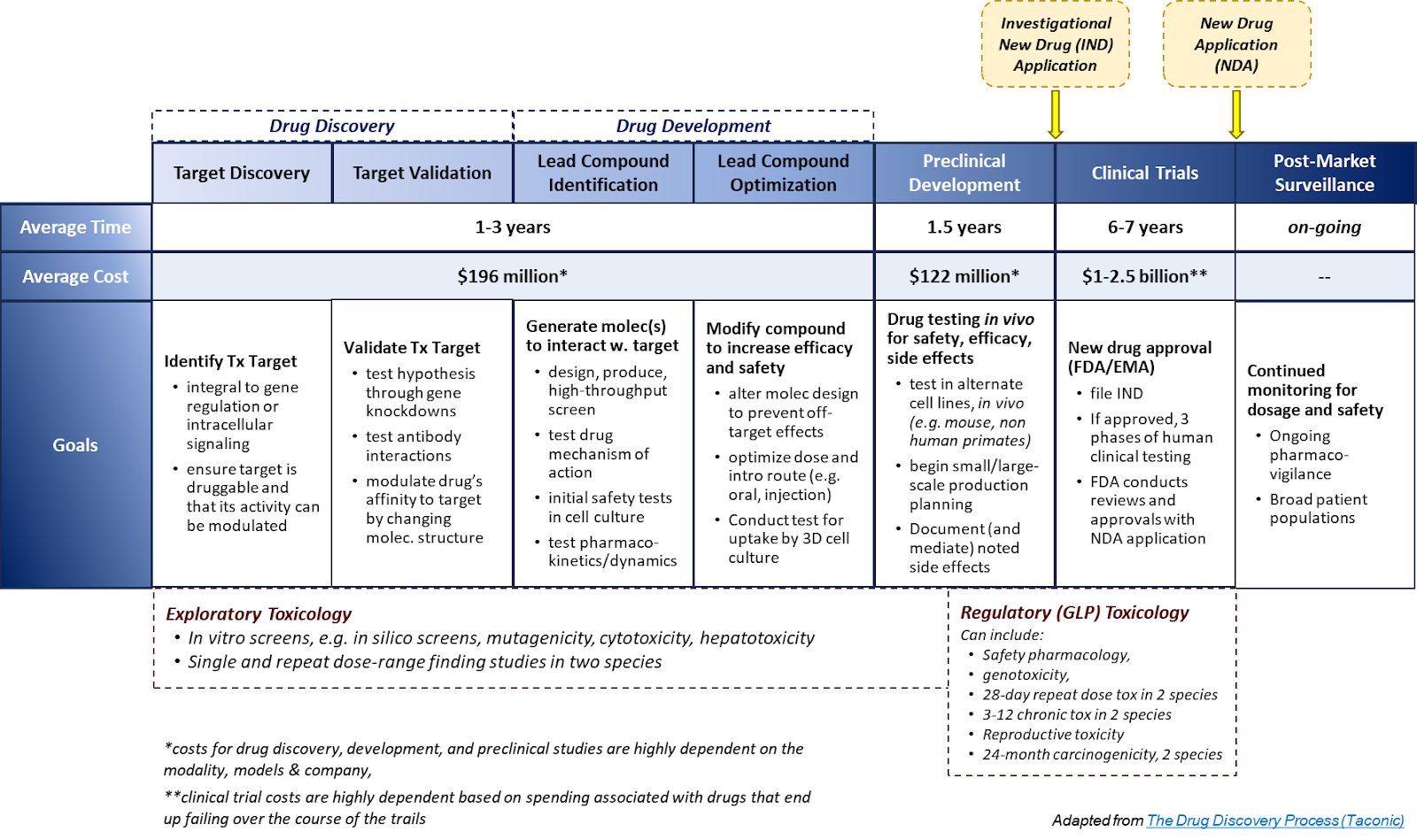
Fig. 1: Discovery & Development to Approval
Fig. 2: Clinical Trial & Approval Pathways
Fig. 3: A Drug’s Three Life Periods
Overview
Target
Selection is an important step of drug discovery defined as the decision to focus on finding an agent with a particular biological action that is anticipated to have therapeutic utility - the decisions in this selection process are influenced by an array of scientific, medical, and strategic considerations…
Example: Genetics / -omics-based drug target identification. Genetics research studies how individual genes or groups of genes are involved in health and disease. Genomics is a study of all of a person’s genes (the genome), including interactions of those genes with each other and with the person’s environment.
Example: Molecular Biology within target selection is the process of locating a biological entity (usually a protein or gene) that interacts with, and whose activity is modulated by, a particular compound.
Validation is the first step in discovering a new drug and can typically take 2-6 months. The process involves the application of a range of techniques that aim to demonstrate that drug effects on the target can provide a therapeutic benefit with an acceptable safety window.
Example: Cell-based studies are the earliest form of in-depth target validation in which researchers test interactions between therapeutic targets and cells in vitro (outside of a living organism, typically in cells in culture).
Example: Animal studies help de-risk later stage clinical development by providing an opportunity for researchers to access in-depth target validation in vivo (inside of a living organism). Commonly used animal models include mice & rats (95% of all laboratory animals), rabbits, zebrafish, and other small mammals including guinea pigs, dogs, cats, & nonhuman primates (NHP). The approval of any new drug for use in humans or animals usually necessitates that toxicity testing be done in at least one small animal (ex. rodents) and one large animal or target species (ex. Dogs, NHPs).
Hit ID
Hit identification involves finding a compound with confirmed activity against a biological target. Hit ID through drug discovery screening can include everything from traditional in vitro approaches to in silico screening technologies. These approaches can be applied in parallel or individually, depending on the nature of the project and the resources available.
High-Throughput Screening: The use of automation to quickly test a large number of samples against a biological target. HTS is frequently used in drug development to rapidly test a large number of potential drugs and look for potential leads that may be starting points for future drug development optimizations.
Example: HTS would be screening a specific library of millions of small molecules for activity in eliminating a target protein.
Assay Development: Assays are procedures for measuring the biological activity of a sample and are often used to test various properties of potential drugs. Assay development is the process of creating assays (or test systems) to evaluate the effectiveness of particular compounds and to identify potential therapeutics. A unique series of assays must be specifically designed for each drug target and indication in question. These assays are then often used in conjunction with High Throughput Screening to test a wide variety of compounds and to begin to generate leads on potential therapeutics.
Example: An example of the process of assay development would be to develop several assays that test the potency, the toxicology, and safety profile of a series of small molecules for targeting a protein implicated in causing cancer.
Lead Optimization: The lead optimization process aims at enhancing the few lead candidates in the previous hit ID step to improve the compounds’ potency, efficacy, reduced off-target effects, and physicochemical properties by selectively modifying the compounds’ molecular structures. Various invitro and in vivo assays are used to understand how the chemical structure impacts different aspects of the drugs’ profiles.
Medicinal Chemistry: During the lead optimization process, medicinal chemistry aims to understand the relationship between the chemical structure and the activity of a compound and analogs (SAR-structure-activity-relationship). Based on this knowledge, a compound's molecular structure will be improved by optimizing the molecules' downstream properties (SPR - Structure-property-relationships).
Example: Target properties that might be improved by modifying the molecular structure include solubility, protein binding, stability, and biological properties.
Initial Animal Efficacy Studies: The potential therapeutic leads and generated analogs must be assessed in relevant animal models to determine their efficacy (meaning how well the compound produces the desired beneficial effects to alleviate a disease or produce a specific outcome). The results from the animal studies should elucidate how the compounds' structural properties and molecule variations will affect efficacy.
Example: In animal models, protein binding, pharmacokinetics as well as metabolite profiles in plasma, urine, and bile would be assessed.
In Vitro & In Vivo ADME: Lead optimization also involves assessing a lead compound's pharmacological properties of Adsorption, distribution, metabolism, and excretion (ADME). Establishing benchmarks of those characteristics will inform the subsequent optimization of the compound’s structure.
Example: In vitro assays include determining the aqueous solubility, lipophilicity, microsomal stability, enzyme inhibition stability, permeability, and hepatotoxicity/cytotoxicity with suitable cell lines.
Pharmacology: Encompassing both in vitro and in vivo assays, this step assesses the pharmacological safety margins and dosing regimes of a lead candidate and can be used to avoid side effects or adverse drug reactions.
Example: In vitro pharmacological safety assays are composed of non-cellular binding assays that establish the IC50/IC90 values (minimum concentration of the drug that is required for 50% or 90% of the maximum effect in vitro). Meanwhile, in vivo assays are used to characterize the ED50 value (drug concentration that produces the desired efficacy in 50% of the studied population or animal model).
Candidate Selection & Profiling
Animal Efficacy: The FDA animal efficacy rule is for the development and testing of compounds, either drugs or biologics, to prevent conditions caused by lethal agents where human trials are not ethical. Four criteria must be met: reasonably well understood mechanism of toxicity, effect is demonstrated in more than one animal species, clear endpoints, and PK/PD data allows for effective dose selection in humans
Example: Potential applications for the animal rule are for vaccinations, plague bacteria, smallpox, and cyanide toxicity
DMPK/ADME (Drug Metabolism and Pharmacokinetics/ Absorption, Dilution, Metabolism, Excretion): Studies done either in vitro or in vivo to allow researchers to make a go/no-go decision in the lead optimization phase of drug discovery. This process helps identify toxicity, supports safety evaluations, provides dosimetry, and indicates drug-drug interactions
Example: In vitro examples can include metabolite profiling, structural elucidation, and drug-drug interactions
(Initial) Formulation & Delivery: The process of creating an efficiency system of delivery that allows the administering of a compound to achieve a therapeutic effect
Example: An osmotically controlled drug delivery system can be used for colon indications
GMP (Good Manufacturing Practices) Synthesis / Manufacturing: System or practice for ensuring that products or compounds are consistently produced to a high standard and controlled according to quality standards.
Example: GMP strategy can be used for new compounds such as in developing antibody-drug conjugates (ADCs) for site-specific production
(Preliminary to Definitive) Rodent Toxicology / Genotoxicology: Rodent toxicology studies are performed as rodents are useful animal test models for investigating toxicity and mechanistic studies. Genotoxicity are studies to determine DNA or chromosomal damage, in which damage may result in cancer
Example: Genotoxicity studies can be performed in rodent models. Mice may be exposed to genotoxic chemicals such as ethyl methanesulfonate to evaluate the toxicity
IND Enabling Studies: Studies conducted to evaluate the potential toxicity, often in animal models, and dosing prior to human trials
Pharmacokinetics (PK) (higher species): Study to understand the fate of the drug from administration to elimination from the body. Understands how the organism affects the drug
Example: PK metrics often include dose, dosing interval, volume of distribution, concentration, absorption half-life, elimination half-life, infusion rate, clearance, bioavailability, and fluctuation
Pharmacodynamic (PD) (Higher Species): Study of the biochemical and physiological effects of drugs or compounds. Understands how the drug affects the organism
Example: PD studies often investigate cell membrane disruption, chemical reactions with downstream effects, interactions with enzymes/structural proteins/carrier proteins/ion channels, and ligand binding to receptors
Toxicology Assessments (Higher Species): Safety evaluation of a compound based on intended uses. Presented as a written summary of potential health effects, dose levels at which health effects may occur, and calculated toxicity values
Example: The process may involve hazard identification, dose-response assessment, exposure assessment, and risk characterization
IND Submission (Investigational New Drug) is a request from a clinical study sponsor to obtain authorization from the FDA to administer an investigational drug or biological product to humans.
Pre-IND Consultation Program: The Pre-Investigational New Drug Application Consultation Program is designed to encourage early communications between the Office of Infectious Diseases (OID) and potential sponsors of new therapeutics for the treatment of infectious diseases.
Example: Pre-IND advice may be requested for the design of nonclinical pharmacology, toxicology and drug activity studies, including discussion on any proposed animal models for the study.
SEND (Standard Exchange of Nonclinical Data): An implementation of the Standard Data Tabulation Model for non clinical studies. This specifies a way to present nonclinical data in a consistent format.
Example: Raw data of toxicology animal studies which are used to support the submission of a new drug to the FDA will be submitted using SEND.
Types of Filing: There are two categories for INDs: Commercial and Research. The main difference is who submits the application to the FDA and the intended purposes for the clinical research.
Commercial: These are generally INDs submitted by a drug company or sponsor as the intended purpose of their clinical research is to collect the data needed to bring the drug to market.
Example: Genentech filing an IND for their drug Vabysmo.
Research (Non-Commercial): These are submitted by physicians and are generally used in cases where the new drug is not being brought to market.
Example: Prove the efficacy of an already approved drug for a new indication or testing a new dosage.
IND Types
Investigator: This IND submitted by a doctor for research on a drug which is not approved or a new indication for an approved drug. The FDA approves the doctor who submits the application to administer the drug and run the investigation.
Treatment: In cases of serious or life-threatening conditions, an experimental drug can be used to treat a patient while the FDA is conducting its review of the drug.
Emergency Use: In times where there is a life-threatening situation and there is no time to wait for an IND the FDA may authorize the use of an experimental drug for patients.
Elements of the Application
Animal Pharmacology & Toxicology Studies: This component should contain information about the preclinical pharmacological and toxicological studies which the sponsor has used to determine that it is reasonably safe to conduct the proposed clinical investigations.
Manufacturing Information: This contains all the information regarding the composition, manufacturer, stability and controls for manufacturing the drug product.
Clinical Protocols & Investigator Information: Detailed protocols for the clinical studies that are being proposed which are used to determine whether the initial-phase trials will expose patients to unnecessary risks. This section also includes information on the qualifications of clinical investigators who are overseeing the administration of the proposed drug.
Data From Any Prior Human Research: Finally the application should include any prior relevant human research using the proposed new drug.
Phase1 - Safety & Dosing: The main goal of Phase 1 clinical trials is to establish the recommended dose and safety of a new drug candidate or drug combinations through testing in 20-80 healthy individuals that are not expected to benefit from the therapy.
Phase 1a: Dose Escalation → MTD
Observed toxicities are classified into dose-limiting toxicities (DLT) and the data is assessed after each cycle of the cohort dosing to determine proceeding. This process is repeated to determine the maximum tolerated dose (MTD), which is the dose level where the DLT is in the range of 25-30%. There are two broad methods of dose escalation: rule-based design and model based designs:
The most common rule-based design is the 3+3, where three patients are enrolled and treated with a dose that is considered safe from preclinical data or similar candidates. If none of the patients are negatively affected, subsequent cohorts are treated with increasing doses. If any patient experiences toxicity, another three patients will be treated with the same dose, and the dose escalation continues until two patients experience DLT.
A common model-based design is the continual reassessment method (CRM). CRM uses a Bayesian approach that integrates the prior patient’s data to estimate the dose and MTD for the next patient. The DLT is updated for each patient until a fixed condition or number of patients reaching the same dose is met.
Phase 1b: Dose Expansion
The addition of a dose-expansion cohort (DEC), often with 12 or more patients, and treating this cohort with the drug candidate at the established maximum tolerated dose (MTD) based on the dose escalation phase. The purpose is to determine whether the recommended Phase II dose is appropriate or if the dose can be lowered.
Phase 2 - Efficacy & Side Effects (Optimal Dosing): Phase 2 clinical trials build on the safety and dosing data gathered during Phase 1 and provide valuable preliminary efficacy data to inform the design of subsequent Phase 3 trials. Phase 2 trials are generally larger than Phase 1 (50 - 500 individuals) and are conducted in both healthy participants as well as individuals with the clinical indication being targeted.
Phase 1b/2a - Optimal dosing strategies:
Phase 1b/2a trials involve establishing the optimal dose and dosing frequency of the investigational drug. Participants may receive the MTD as established within Phase 1 trials. In Phase 1b/2a, patients from the targeted indication may be involved and these studies are often the first randomized and controlled trials. The primary goal of Phase 1b/2a is to establish the pharmacokinetics and pharmacodynamics associated with the dosing strategies studied.
Phase 2a - Safety, tolerability, pharmacokinetics, & pharmacodynamics:
Phase 2a trials are designed to assess the safety and tolerability (including side effects) associated with the chosen dosing strategies for an investigational drug. Phase 2a trials are typically larger than Phase 1 studies and involve patients with the disease indication of interest. Phase 2a may also be controlled and blinded, providing preliminary efficacy data.
Phase 2b - Preliminary efficacy studies:
Phase 2b trials aim to establish initial efficacy data for the use of the investigational drug at the optimal dosing strategies established in the prior Phase 1b/2a studies.
Phase 2b studies generally involve 50 up to a few hundred participants, randomized and with a control arm. Disease progression is commonly used as the primary endpoint in these studies, the overall outcome of which is used to determine whether to progress to Phase 3 and inform trial design for subsequent Phase 3 studies.
The size of Phase 2b studies is sufficient to provide preliminary efficacy data, but underpowered to accurately assess efficacy, which will be tested during Phase 3 trials. These larger trials provide safety data, adding to that collected during Phase 1.
FDA Consultation for Phase 3 trial design: Following conclusion of Phase 2 clinical trials, investigators and sponsors may seek advice from FDA on Phase 3 clinical trials. These consultations involve studying the preliminary data from Phases 1 and 2, in addition to the original IND, and are designed to establish the potential to progress to Phase 3 and the design for Phase 3 trials. Advice may be sought around trial size, outcomes and endpoints, data collection, plans for analysis of collected data, and relevant safety concerns.
Phase 3 - Comparative Efficacy & ADE Monitoring: The purpose of Phase 3 clinical trials is to establish whether a therapeutic treatment is safe and effective (or more effective) relative to the current standard of care at a statistically significant level.
Phase 2b/3a: The clinical trial phases that progress from refining appropriate dose to beginning to test the wide-scale efficacy of the therapeutic in treating, diagnosing, or preventing the target disease while introducing no unacceptable safety concerns.
Phase 2b usually includes up to a couple hundred patients and, if successful, provides a starting point for carrying out a phase 3a study.
Phase 3a: A pivotal study, including several hundred to several thousand patients, with the aim of obtaining statistically significant evidence supporting the efficacy of a treatment relative to the current standard of care, as well as its safety. Close attention is paid to Adverse Drug Effects (ADE) across the treatment group.
Studies are designed to support the claims of the company producing the therapeutic or claims intended for the patient information leaflet (PIL).
Types of studies commenced during 3a may include:
Non-inferiority trials - demonstrates the new treatment is better and/or safer than an existing treatment.
Long-term safety studies - A study that focuses on long term safety, and may be designed to understand risk across various populations.
The results during phase 3 trials are essential in risk-benefit analysis that informs the ultimate approval of an NDA by regulatory authorities.
Not designed to provide information about pricing or marketing of the therapeutic.
NDA Preparation (New Drug Application): The NDA is a formal proposal by the drug manufacturer to gain FDA approval for a new drug.
Considerations
Regulatory Pathway
The regulatory pathway through which the NDA is submitted dictates how much and what information needs to be included.
There are 3 Major Pathways:
505(b)(1) or “Stand-alone” NDA: Used for new drugs and must include all safety and efficacy studies conducted by the applicant
505(j) or abbreviated NDA (ANDA): Used for the approval of generic drugs and demonstrates biosimilarity to existing approved drugs.
505(b)(2) or “Hybrid Application”: Uses pre-existing studies and data (i.e. not conducted by the applicant) to expedite approval process. Typically reserved for modifications to previously approved drugs, including changes in dosage, delivery, formulation, and/or chemistry.
Expedited Development and/or Review: The FDA provides four pathways for accelerated approval to market. These pathways are designed for therapies that fill significant unmet needs for serious conditions.
Priority Review
Priority Review expedites the FDA’s reviewal process, decreasing time to decision from 10 months to 6 months.
Breakthrough Therapy
Breakthrough designation is provided to therapies which represent a significant improvement over existing SOC
Accelerated Approval
Accelerated approval is given to therapies for serious conditions that need to be approved using surrogate endpoints
Fast Track
Fast track facilitates the development and review of drugs to treat serious conditions and fill unmet medical needs.
Elements
Drug Ingredients: Full description of the drug’s components and how it behaves in the body.
Animal Studies: Full description of the animal studies using the drug
Clinical Trials: Full description of the human clinical trials using the drug.
Manufacturing, Processing, & Packaging: Detailed description of the facilities, methods, and controls used to create the drug as well as all components of the label.
Phase 3b: Phase 3b trials occur following the submission of an NDA for regulatory approval. The data collected are not needed for approval, but often can be used to bolster or support the application.
Approval & Commercialization
After the drug has passed the NDA before, it is now approved as a new pharmaceutical for sale and marketing in the U.S.
Pricing: The process of determining the new drug’s price.
Usually when pharmaceutical companies price their new drug, they consider a drug’s uniqueness and effectiveness as well as competitions from other companies. They also consider the research and development cost that went into the drug.
Marketing: The process of promoting and persuading a new drug for prescription and over-the-counter sale.
The pharmaceutical companies would do market research to understand the audience they want to reach, visit doctors to pitch their drugs, provide free promotional samples, advertise through journals and conferences and direct consumer advertising.
Distribution: The process of delivering the drug to the customers
The pharmaceutical companies typically develop a conceptual framework to illustrate the flows of goods, services and funds among system participants, and then calculate the most efficient, cost-effective distribution channels for their new prescription drug.
Phase 4 - Observational Study (Post Marketing Safety & Efficacy):
Phase 4 clinical trials take place after a new treatment has been approved by the FDA and is on the market. The goal is to determine if there are rare unknown side effects, as well as better understand the long term implications of the drug. The drugs are available for use and patients can take the drug without participating in the phase 4 clinical trial.
IND-Enabling Studies
(Nuventra, 2021) Key IND-Enabling Studies
(Charles River) IND-Enabling Studies
IND Submission
Applicable Regulations
21CFR Part 201: Drug Labeling
21CFR Part 312: Investigational New Drug Application
21CFR Part 314: INDA and NDA Applications for FDA Approval to
Market a New Drug (New Drug Approval)
21CFR Part 316: Orphan Drugs
21CFR Part 50: Protection of Human Subjects
21CFR Part 54: Financial Disclosure by Clinical Investigators
21CFR Part 56: Institutional Review Boards
21CFR Part 58: Good Lab Practice for Nonclinical Laboratory
[Animal] Studies
Clinical Trials
Additional Resource Links
Why Product Lifecycle Management Fails Pharma: Time for a Rethink? (Bernard Associates, 2013)
Cost of Developing a New Drug (Tufts, 2016)
After 60 years, scientists uncover how thalidomide produced birth defects (Dana Farber, 2021)
Promoting Safe and Effective Drugs for 100 Years (FDA Consumer Magazine, 2006)
The Drug Development Process (Taconic, 2019)
Alix Ventures, by way of BIOS Community, is providing this content for general information purposes only. Reference to any specific product or entity does not constitute an endorsement nor recommendation by Alix Ventures, BIOS Community, or its affiliates. The views & opinions expressed by guests are their own & their appearance on the program does not imply an endorsement of them nor any entity they represent. Views & opinions expressed by Alix Ventures employees are those of the employees & do not necessarily reflect the view of Alix Ventures, BIOS Community, affiliates, nor its content sponsors.
Join BIOS Community 🎉
Become a member, continue the conversation, connect with like-minded Life Science innovators, access exclusive resources, & invite-only events…
Apply to Join — Membership Application
For More Interesting Content 💭
🧬 Podcast — Stream Full Episodes
🧪 YouTube — Watch Videos
🩺 Twitter — Explore Feed
🦠 LinkedIn — Read Posts